Nucleic Acid Amplification Test Naat From A Clia Certified Laboratory
Lower respiratory secretions such as sputum and bronchoalveolar lavage fluid are also used if a patient has pneumonia or lung involvement with infection.
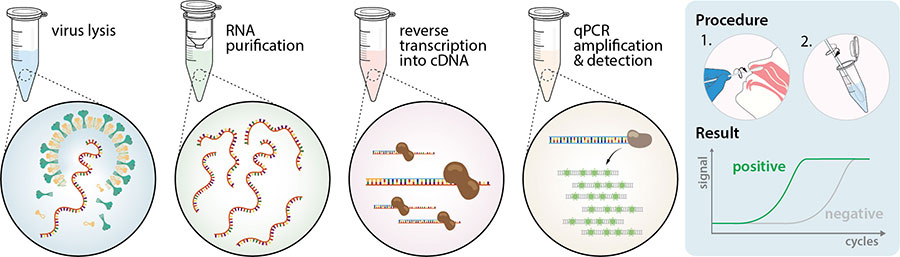
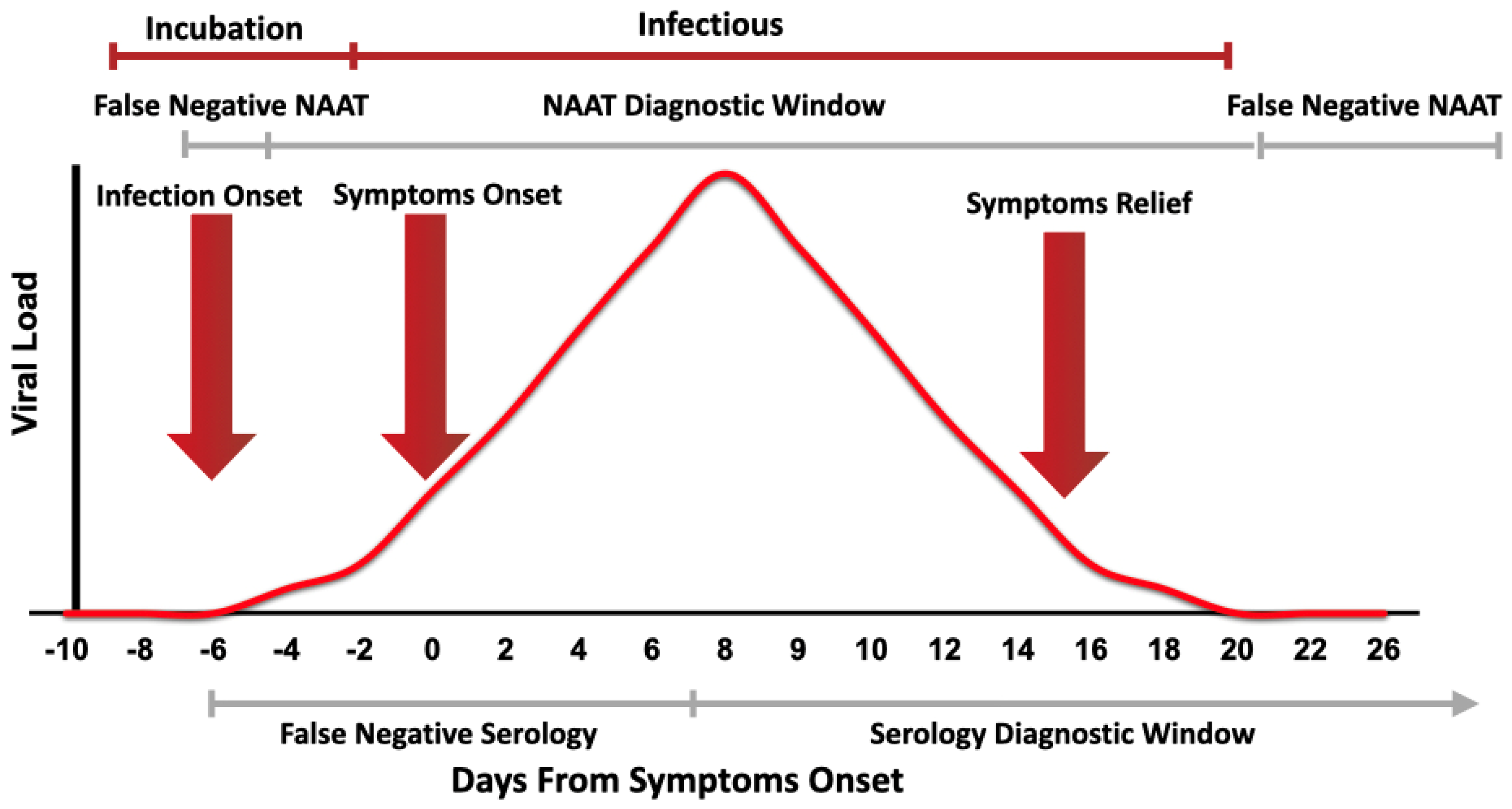
Nucleic acid amplification test naat from a clia certified laboratory. However only a certain covid 19 test will be accepted which is the nucleic acid amplification test naat. Nucleic acid amplification testing requires respiratory samples from the patient because sars cov 2 is a respiratory virus. As tests are submitted quest will route the incoming specimens to a molecular pcr test ldt roche hologic or others to optimize capacity and minimize the possibility of a backlog on a given platform. Diagnostic test viral test molecular test nucleic acid amplification test naat rt pcr test.
According to the. A single test code will allow quest to better manage the capacity within each lab and across labs performing the molecular pcr covid 19 tests. Unlike other tests this naat test doesn t involve taking a blood sample throat sample or saliva test. To help guests prepare for travel to the islands.
Originally the test had to be an naat test nucleic acid amplification test performed at a clia certified lab. This test is intended to be performed on respiratory specimens collected from individuals who meet the centers for diseases control and prevention cdc clinical and or. In fall 2012 the association of state and territorial health officials astho recognized the use of a universal laboratory screening test for multidrug resistant tuberculosis mdr tb. Clia to perform high complexity testing and who create laboratory developed tests.
The test must be. The covid 19 test for travel has been granted eua emergency use authorization by the fda and is known as a naat nucleic acid amplification test. Alaska is offering safe and reliable testing options. Qualifying tests must be nucleic acid amplification tests also known as naat a molecular test that requires a minimally invasive nasal swab sample or saliva it reported.
The test must be an fda authorized nucleic acid amplification test naat performed using a nasal swab and processed by a clia certified laboratory. Nasopharyngeal swabs are most commonly used. That was changed in early october to be testing places only in the trusted.