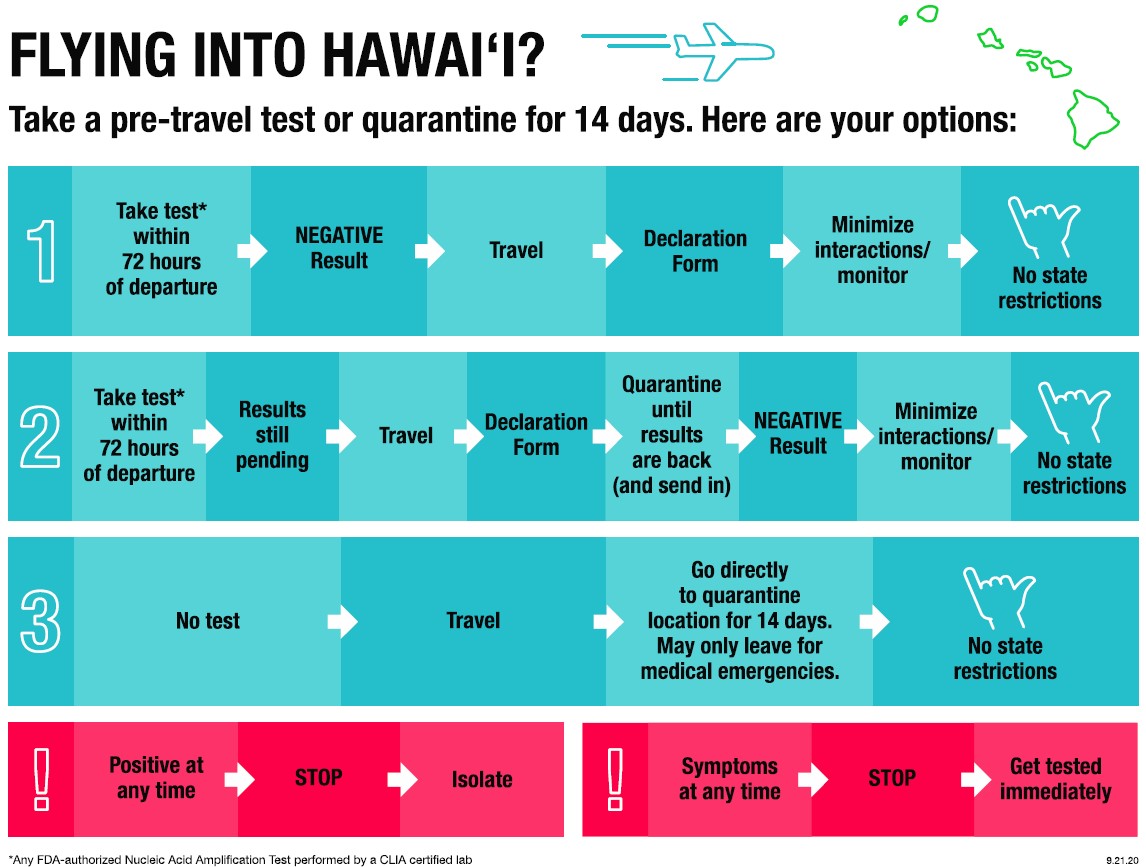
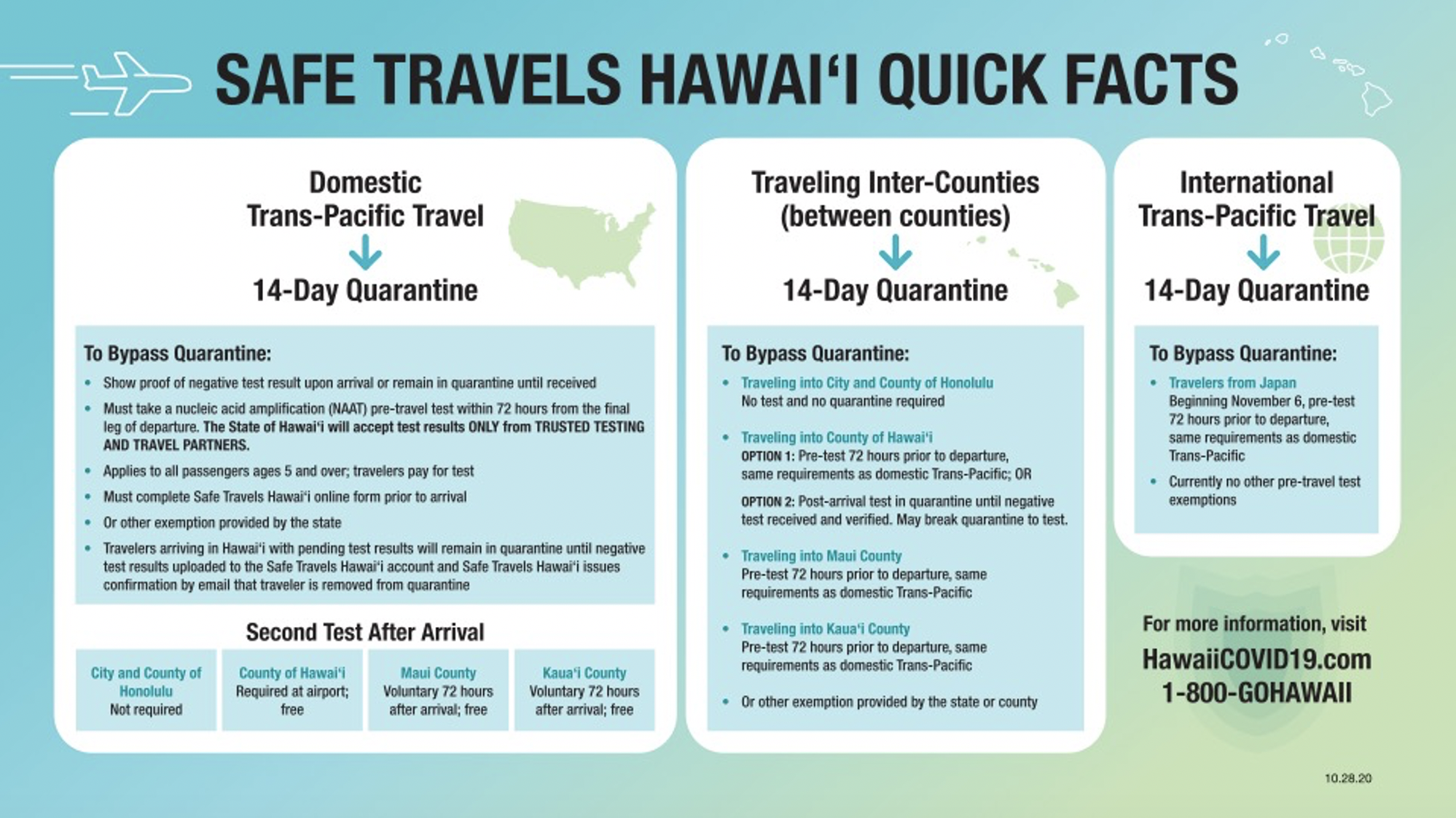
Nucleic Acid Amplification Test Naat From A Clia Certified Laboratory Seattle

This is a very sensitive and definitive test that looks for the rna of the corona virus using a technique called rt pcr.
Nucleic acid amplification test naat from a clia certified laboratory seattle. In seattle alaska airlines. Unlike other tests this naat test doesn t involve taking a blood sample throat sample or saliva test. This test intended for the qualitative detection of nucleic acid from sars cov 2 in nasopharyngeal oropharyngeal swabs and nasal swabs. Lower respiratory secretions such as sputum and bronchoalveolar lavage fluid are also used if a patient has pneumonia or lung involvement with infection.
A nucleic acid amplification test naat for sars cov 2 covid 19 this testing looks for viral rna in a nasal swab specimen a positive result indicates an active infection. As tests are submitted quest will route the incoming specimens to a molecular pcr test ldt roche hologic or others to optimize capacity and minimize the possibility of a backlog on a given platform. 6 5 2020 covid 19 testing guidance nucleic acid amplification pcr testing 6 5 2020 updates from prior version 1. Instead the covid 19 test for travel is a nasal swab test that s administered by a.
The covid 19 test for travel has been granted eua emergency use authorization by the fda and is known as a naat nucleic acid amplification test. Nasopharyngeal swabs are most commonly used. However only a certain covid 19 test will be accepted which is the nucleic acid amplification test naat. The test must be an fda authorized nucleic acid amplification test naat performed using a nasal swab and processed by a clia certified laboratory.
Testing may be considered for asymptomatic close contacts of persons with covid 19. Nucleic acid amplification testing requires respiratory samples from the patient because sars cov 2 is a respiratory virus. Alaska is offering safe and reliable testing options. Diagnostic test viral test molecular test nucleic acid amplification test naat rt pcr test lamp test rapid diagnostic test some molecular tests are also rapid tests.
Administration authorized nucleic acid amplification test naat from a certified federal certified laboratory improvement amendments lab then follows a lost of. Added guidance for covid testing of patients without covid 19 symptoms.