Nucleic Acid Amplification Test Naat For Covid 19

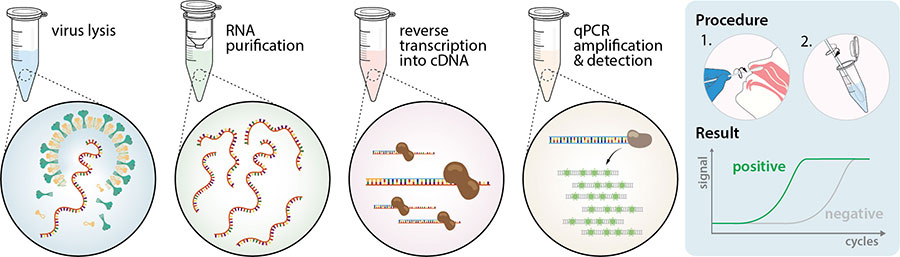
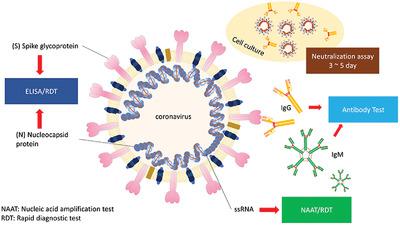
Sars cov 2 rna covid 19 qualitative naat the sars cov 2 rna covid 19 nucleic acid amplification test naat is a qualitative multi target molecular diagnostics test that aids in the detection of covid 19.
Nucleic acid amplification test naat for covid 19. Covid 19 testing guidance version 1 72 revised. According to the. The proportion of true infections that will be detected by the test as a positive test. The incubation period for covid 19 is generally 2 7 days up to 12 most often 4 5 days china singapore example.
Nucleic acid amplification testing requires respiratory samples from the patient because sars cov 2 is a respiratory virus. How does the test work. The covid 19 treatment guidelines panel the panel recommends that a nucleic acid amplification test naat for severe acute respiratory syndrome coronavirus 2 sars cov 2 be used to diagnose acute infection aiii. Nucleic acid amplification testing naat gold standard.
Lower respiratory secretions such as sputum and bronchoalveolar lavage fluid are also used if a patient has pneumonia or lung involvement with infection. The test code is 39448. Interpreting the results of nucleic acid amplification testing nat. 6 5 2020 covid 19 testing guidance nucleic acid amplification pcr testing 6 5 2020 updates from prior version 1.
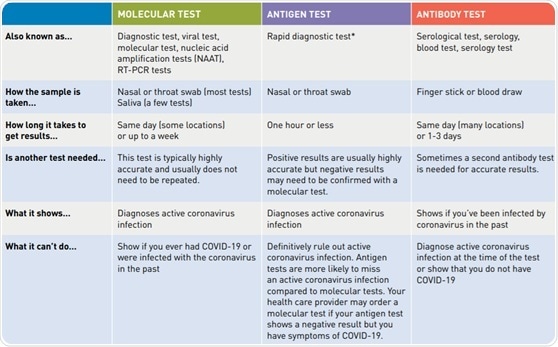
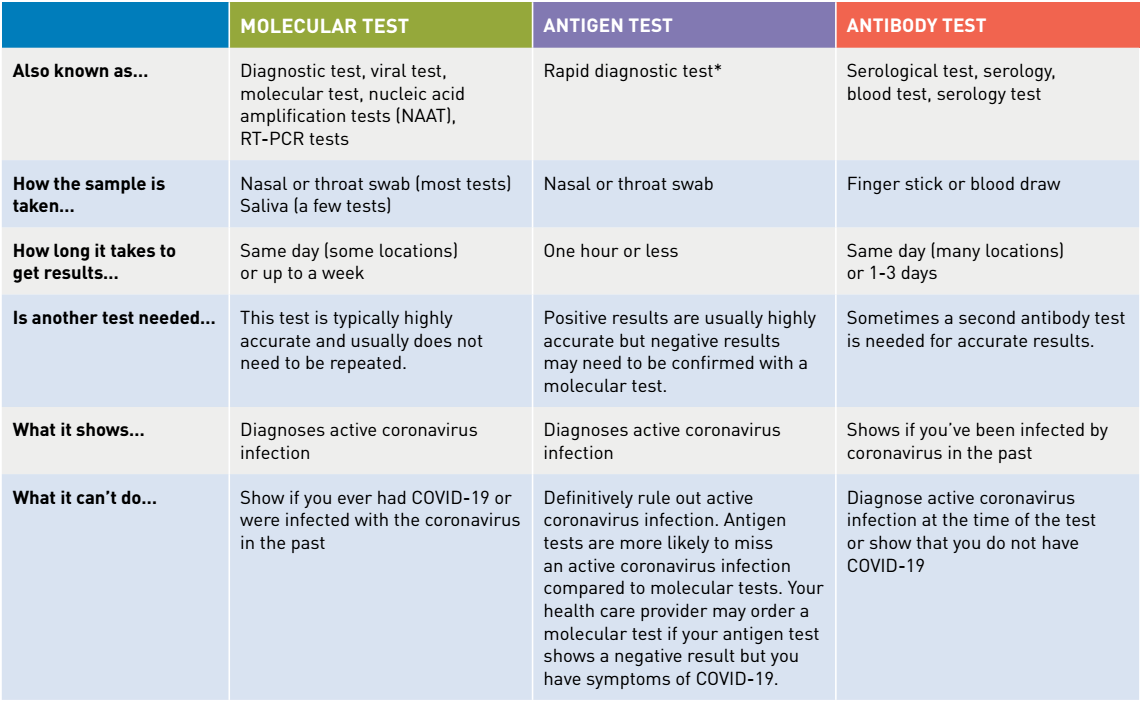
Diagnostic test viral test molecular test nucleic acid amplification test naat rt pcr test lamp test. The molecular tests have been authorized only for the detection of nucleic acid from sars cov 2 not for any other viruses. The nat works by detecting rna specific to the sars cov 2 virus that causes covid 19 infection after rna has been extracted from the specimen and then amplified in the laboratory. Testing may be considered for asymptomatic close contacts of persons with covid 19.
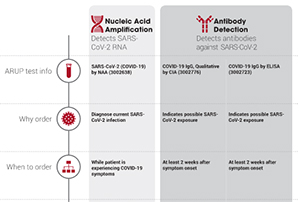
A doctor pharmacist or other health professional orders a covid 19 test. Or pcr tests for covid 19 in the respiratory tract april 30 2020 1. However only a certain covid 19 test will be accepted which is the nucleic acid amplification test naat. Results for the test must come from a clia certified laboratory.
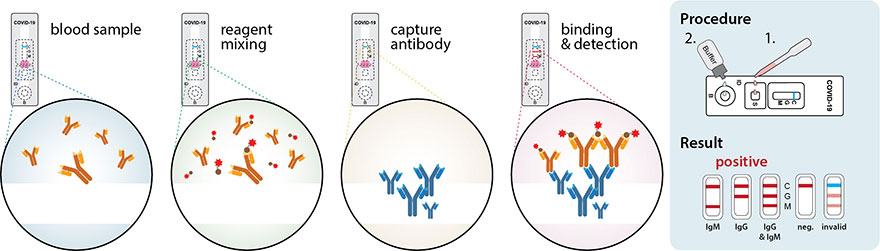
The panel recommends against the use of serologic testing as the sole basis for diagnosis of acute sars cov 2 infection aiii. Added guidance for covid testing of patients without covid 19 symptoms. The test name is sars cov 2 rna covid 19 qualitative naat.