Nucleic Acid Amplification Test Naat Covid

Nucleic acid amplification tests naats designed to detect one or more gene sequences specific to sars cov 2 are essential for confirming covid 19 diagnoses.
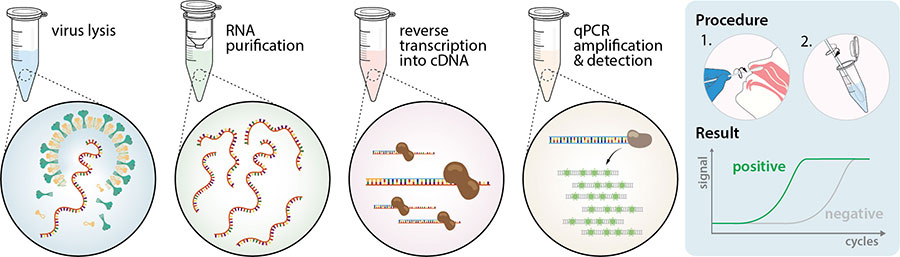
Nucleic acid amplification test naat covid. Lower respiratory secretions such as sputum and bronchoalveolar lavage fluid are also used if a patient has pneumonia or lung involvement with infection. The nat works by detecting rna specific to the sars cov 2 virus that causes covid 19 infection after rna has been extracted from the specimen and then amplified in the laboratory. 6 5 2020 covid 19 testing guidance nucleic acid amplification pcr testing 6 5 2020 updates from prior version 1. This type of testing also called molecular or viral testing is done by swabbing the nose or mouth or collecting saliva.
Sars cov 2 rna covid 19 qualitative naat the sars cov 2 rna covid 19 nucleic acid amplification test naat is a qualitative multi target molecular diagnostics test that aids in the detection of covid 19. Nucleic acid amplification testing requires respiratory samples from the patient because sars cov 2 is a respiratory virus. The test name is sars cov 2 rna covid 19 qualitative naat. The first detects viral rna using molecular methods such as polymerase chain reaction pcr.
Indeterminate test result was not conclusive and test should be repeated. The test code is 39448. According to the. This test is intended to be performed on respiratory specimens collected from individuals who meet the centers for diseases control and prevention cdc clinical and or.
How does the test work. Polymerase chain reaction pcr or nucleic acid amplification test naat. Covid 19 testing guidance version 1 72 revised. All test specific information can be found in the test directory.
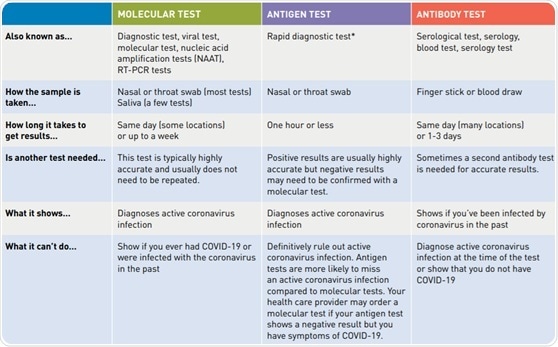
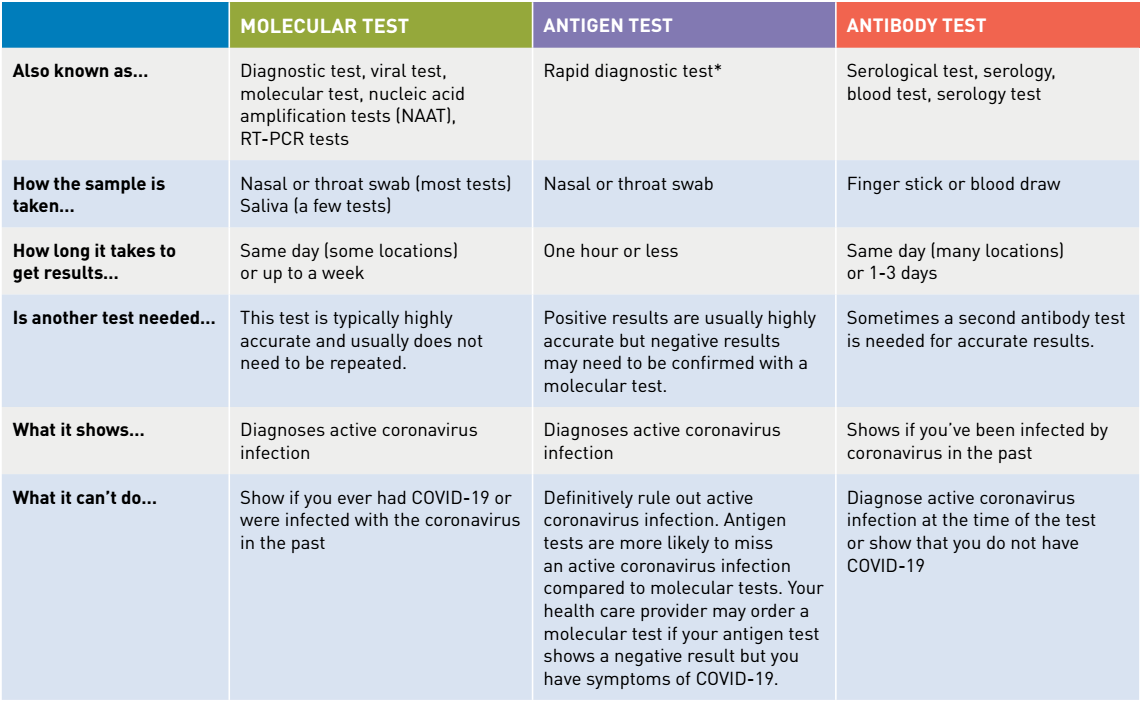
Or pcr tests for covid 19 in the respiratory tract april 30 2020 1. Nucleic acid amplification testing naat there are two main types of tests for covid 19. Interpreting the results of nucleic acid amplification testing nat. Added guidance for covid testing of patients without covid 19 symptoms.
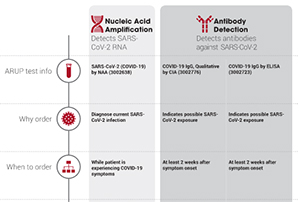
You can read more about this test in arup consult s covid 19 topic. On february 4 th 2020 the united states u s secretary of health and human services announced that circumstances existed justifying authorization of the emergency use of sars cov 2 molecular tests. Results for the test must come from a clia certified laboratory. Nasopharyngeal swabs are most commonly used.
However only a certain covid 19 test will be accepted which is the nucleic acid amplification test naat.