Nucleic Acid Amplification Test Naat Covid Cost
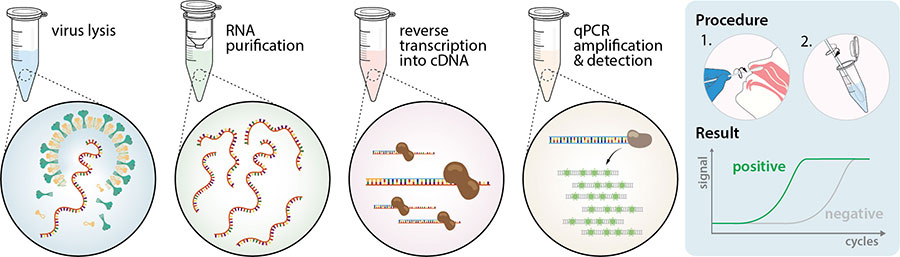
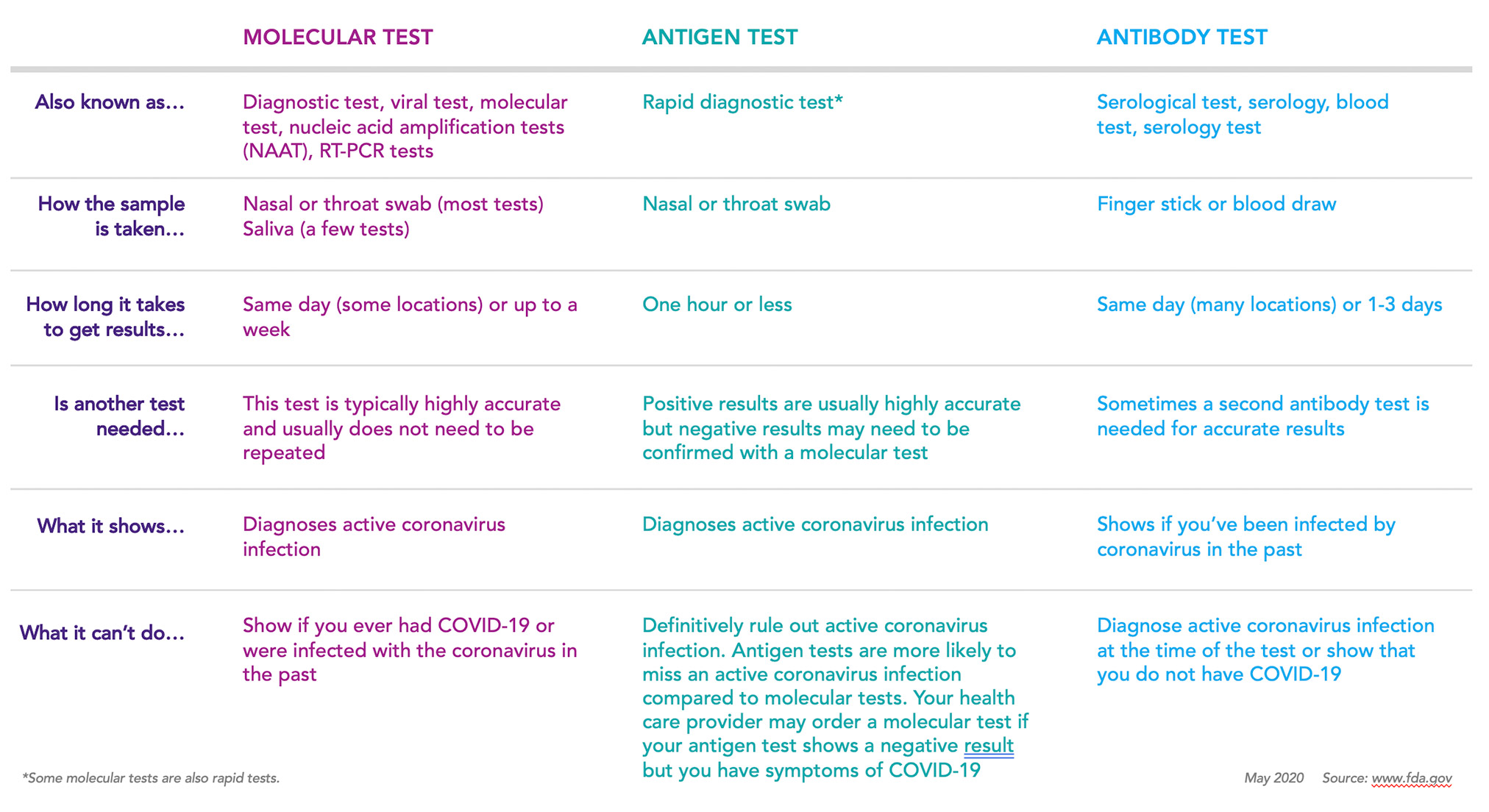
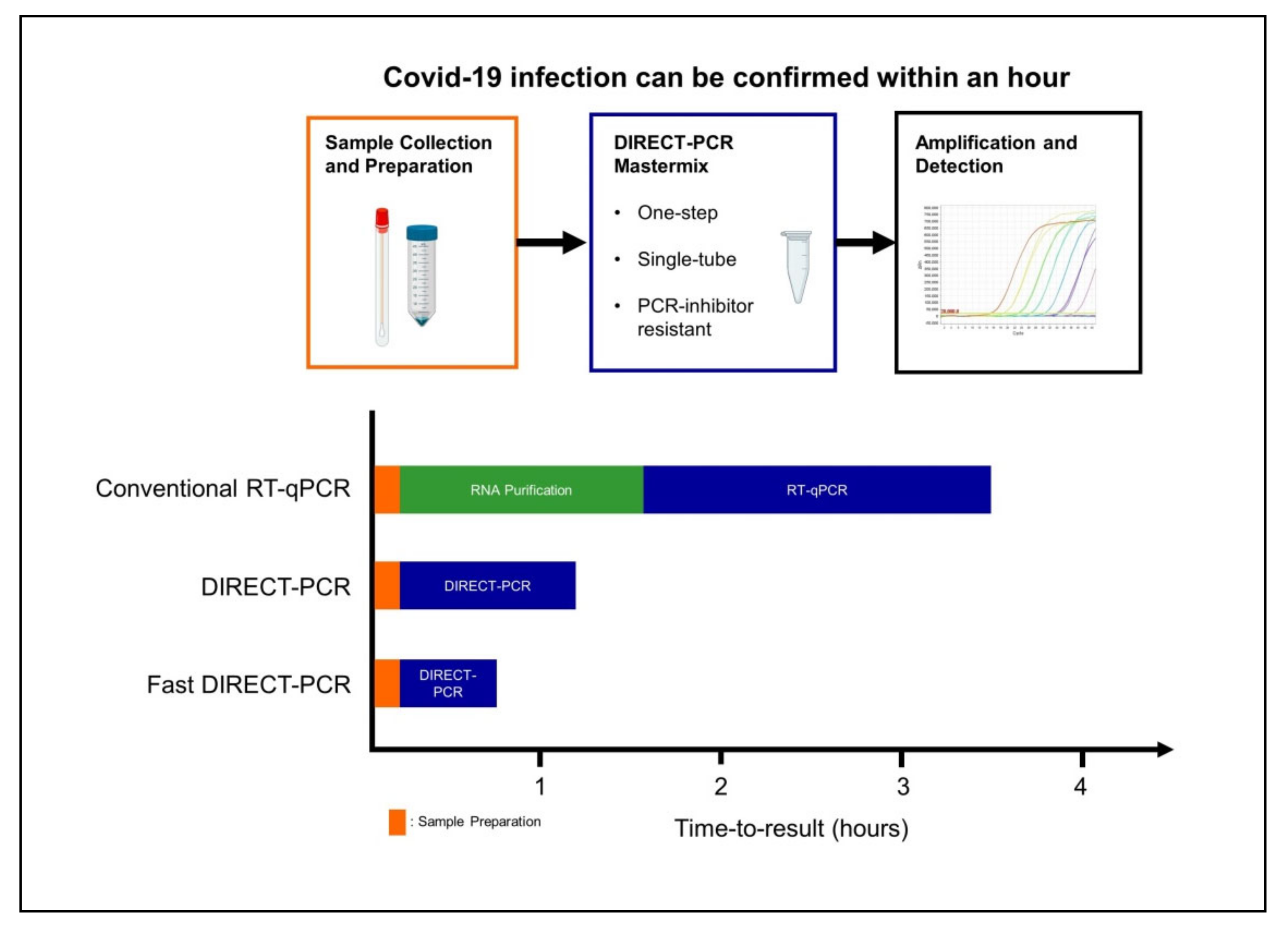
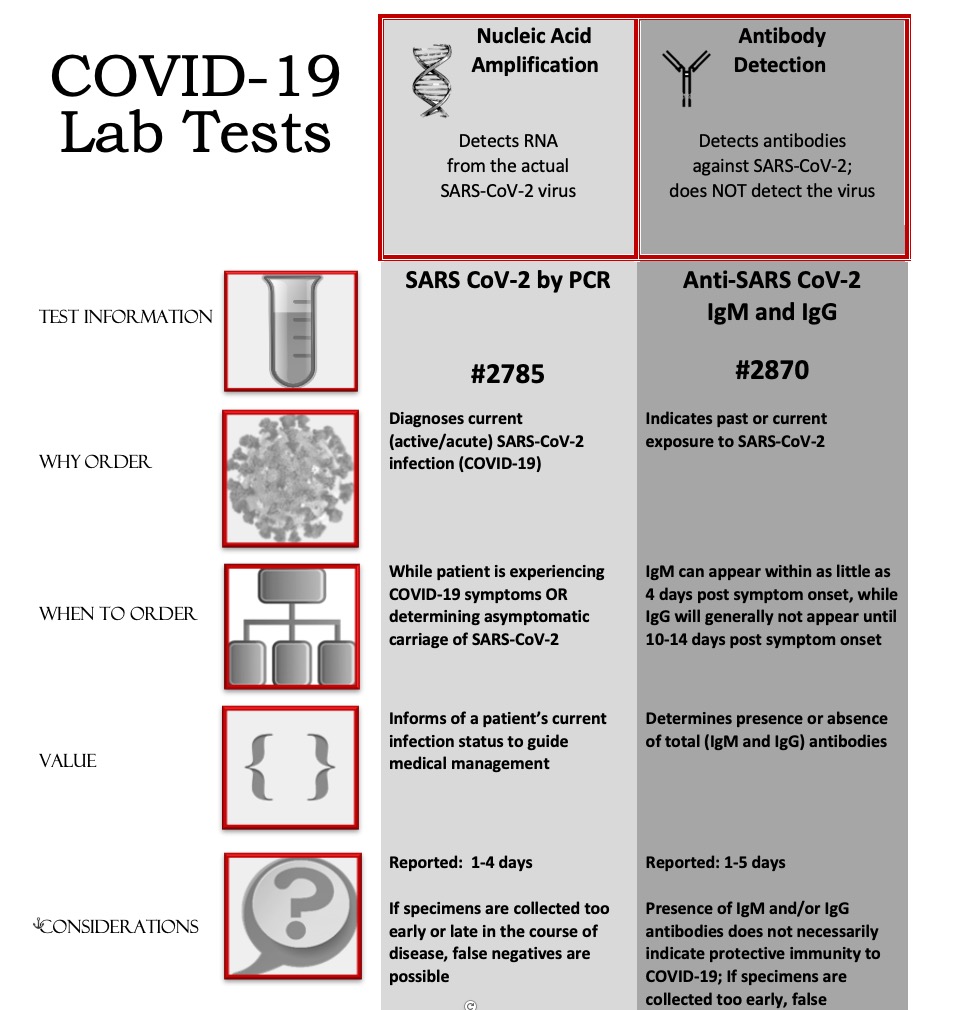
The nat works by detecting rna specific to the sars cov 2 virus that causes covid 19 infection after rna has been extracted from the specimen and then amplified in the laboratory.
Nucleic acid amplification test naat covid cost. Sars cov 2 rna covid 19 qualitative naat the sars cov 2 rna covid 19 nucleic acid amplification test naat is a qualitative multi target molecular diagnostics test that aids in the detection of covid 19. Initially the cost of the test was. How does the test work. Based nucleic acid amplification test.
The rt pcr test is also the most expensive of all those used for covid 19. Naat machines need an. The test code is 39448. The covid 19 test for travel has been granted eua emergency use authorization by the fda and is known as a naat nucleic acid amplification test.
This type of testing may also be known as viral testing or nucleic acid amplification testing naat. The test name is sars cov 2 rna covid 19 qualitative naat. The best way to get a coronavirus test is to contact your health care provider. Results for the test must come from a clia certified laboratory.
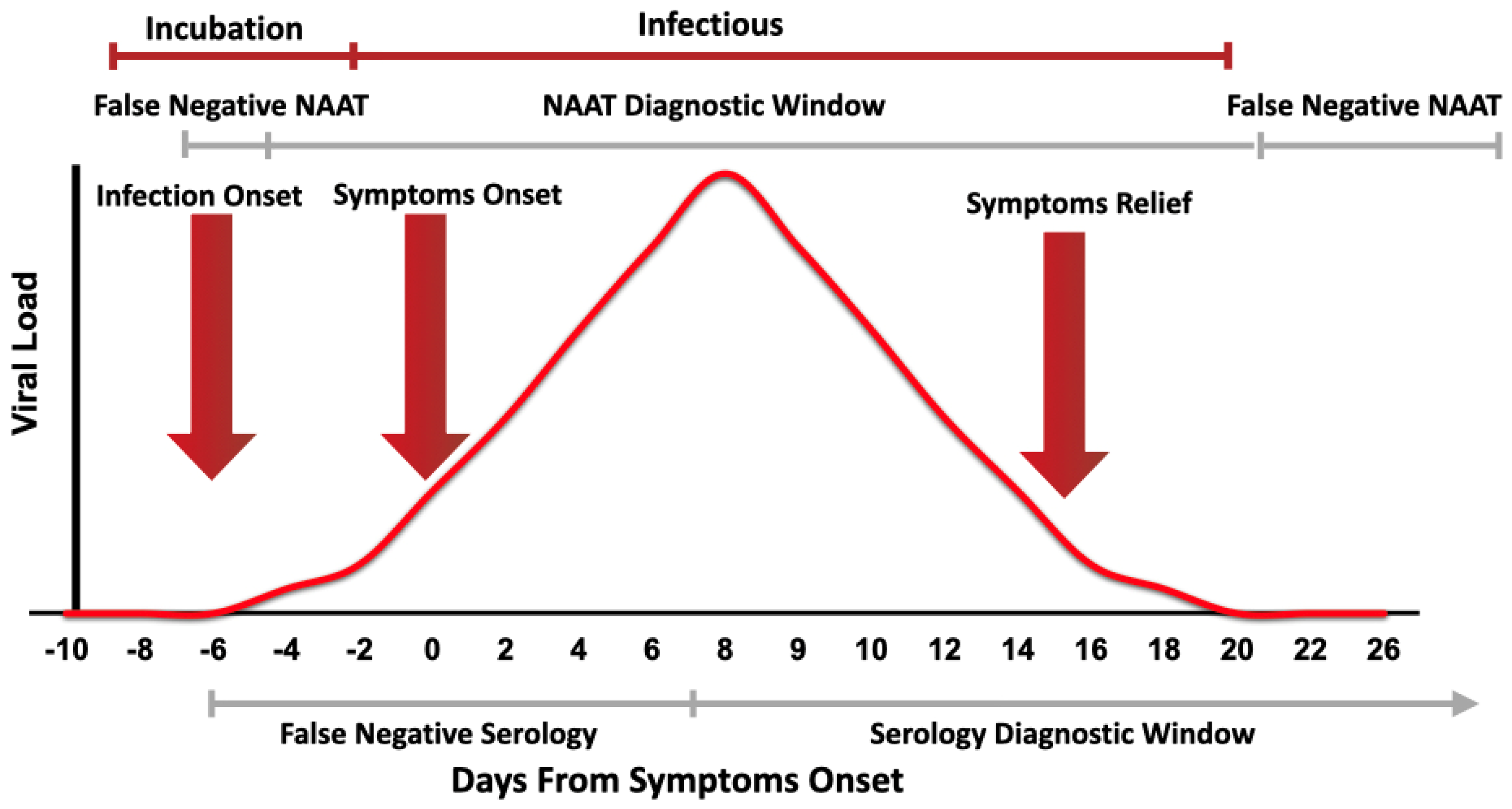
In partnership with alaska airlines and bridge diagnostics bartell drugs is offering covid 19 molecular testing via rt pcr for screening of symptom free travelers. This test is intended to be performed on respiratory specimens collected from individuals who meet the centers for diseases control and prevention cdc clinical and or. Interpreting the results of nucleic acid amplification testing nat. Added guidance for covid testing of patients without covid 19 symptoms.
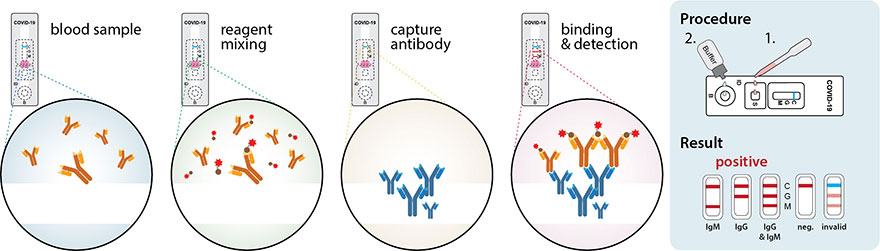
Unlike other tests this naat test doesn t involve taking a blood sample throat sample or saliva test. However only a certain covid 19 test will be accepted which is the nucleic acid amplification test naat. The molecular tests have been authorized only for the detection of nucleic acid from sars cov 2 not for any other viruses. 6 5 2020 covid 19 testing guidance nucleic acid amplification pcr testing 6 5 2020 updates from prior version 1.
Or pcr tests for covid 19 in the respiratory tract april 30 2020 1. Testing may be considered for asymptomatic close contacts of persons with covid 19.