Nucleic Acid Amplification Test Naat Covid 19
Polymerase chain reaction pcr or nucleic acid amplification test naat.
Nucleic acid amplification test naat covid 19. The test code is 39448. Nucleic acid amplification tests are used to diagnose covid 19. Testing may be considered for asymptomatic close contacts of persons with covid 19. Nucleic acid amplification tests.
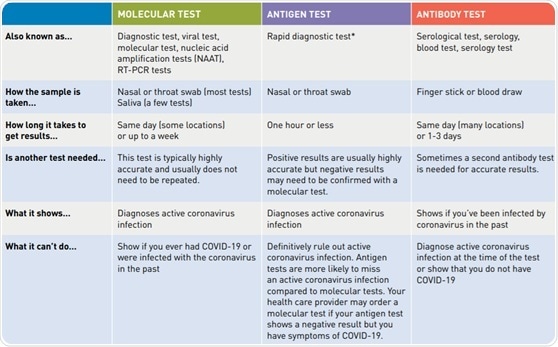
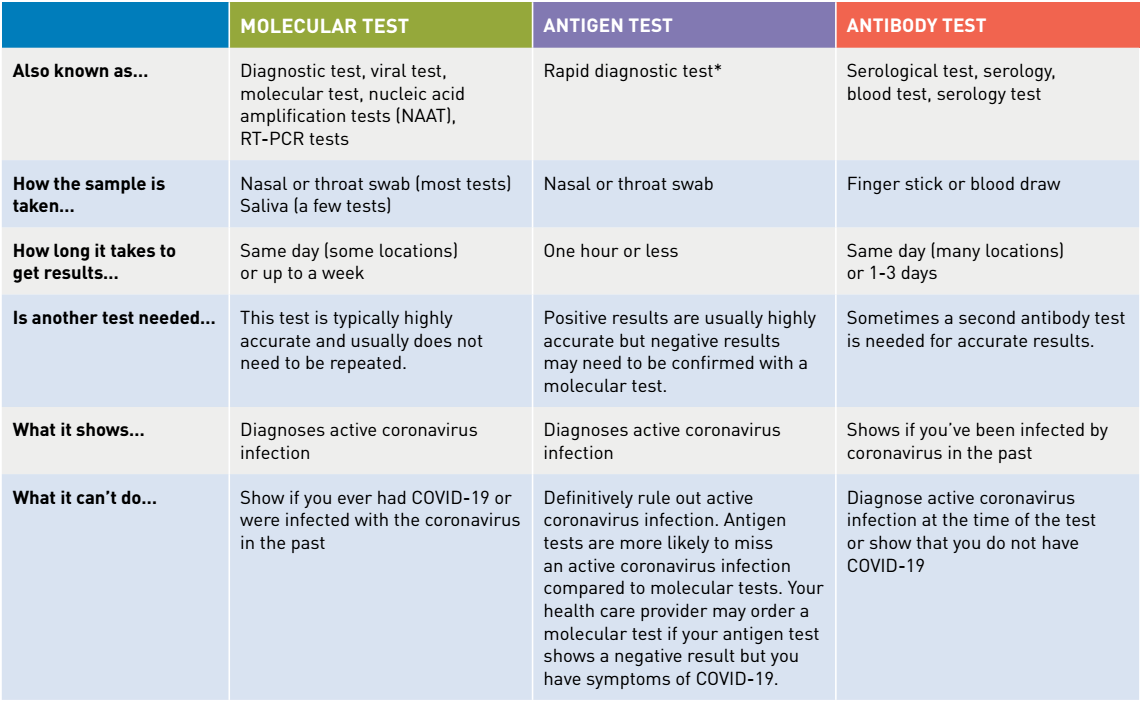
Authorized assays for viral testing include those that detect sars cov 2 nucleic acid or antigen. Or pcr tests for covid 19 in the respiratory tract april 30 2020 1. The molecular tests have been authorized only for the detection of nucleic acid from sars cov 2 not for any other viruses. 6 5 2020 covid 19 testing guidance nucleic acid amplification pcr testing 6 5 2020 updates from prior version 1.
However only a certain covid 19 test will be accepted which is the nucleic acid amplification test naat. A positive test does not necessarily mean that the person is still contagious with the virus. Sars cov 2 rna covid 19 qualitative naat the sars cov 2 rna covid 19 nucleic acid amplification test naat is a qualitative multi target molecular diagnostics test that aids in the detection of covid 19. Covid 19 testing guidance version 1 72 revised.
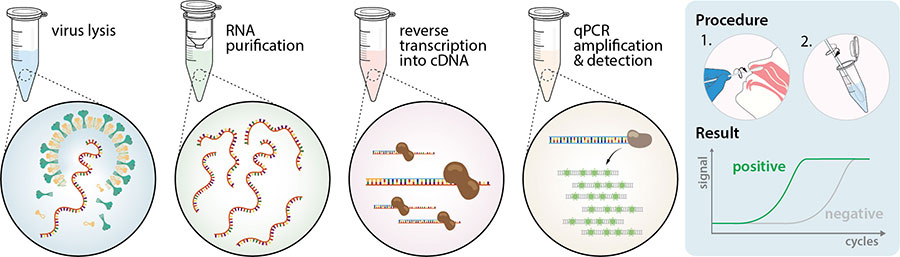
Nucleic acid amplification testing requires respiratory samples from the patient because sars cov 2 is a respiratory virus. It is an automated assay that utilizes isothermal nucleic acid amplification technology. They determine whether a person is currently infected by looking for the presence of viral genetic material. Viral nucleic acid or antigen tests check samples from the respiratory system such as nasal or oral swabs or saliva to determine whether an infection with sars cov 2 the virus that causes covid 19 is present.
Added guidance for covid testing of patients without covid 19 symptoms. The test name is sars cov 2 rna covid 19 qualitative naat. This test is intended to be performed on respiratory specimens collected from individuals who meet the centers for diseases control and prevention cdc clinical and or. Interpreting the results of nucleic acid amplification testing nat.
Results for the test must come from a clia certified laboratory. All test specific information can be found in the test directory. The nat works by detecting rna specific to the sars cov 2 virus that causes covid 19 infection after rna has been extracted from the specimen and then amplified in the laboratory. And a negative test suggests the symptoms are not due to the covid 19 virus.
This type of testing. For sars cov 2 the test identifies specific sequences of sars cov 2 rna the virus s genetic material. Lower respiratory secretions such as sputum and bronchoalveolar lavage fluid are also used if a patient has pneumonia or lung involvement with infection. Nasopharyngeal swabs are most commonly used.