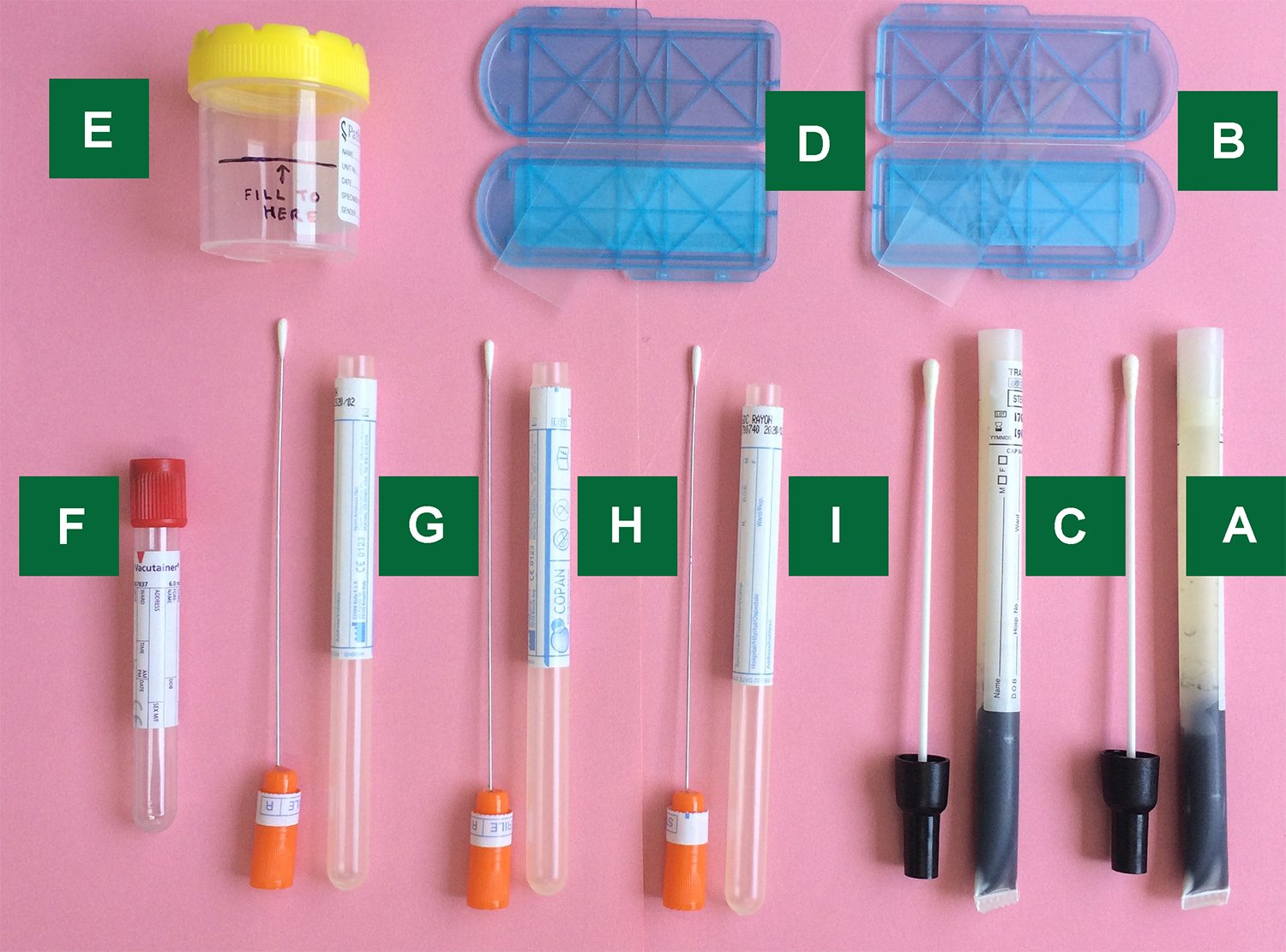
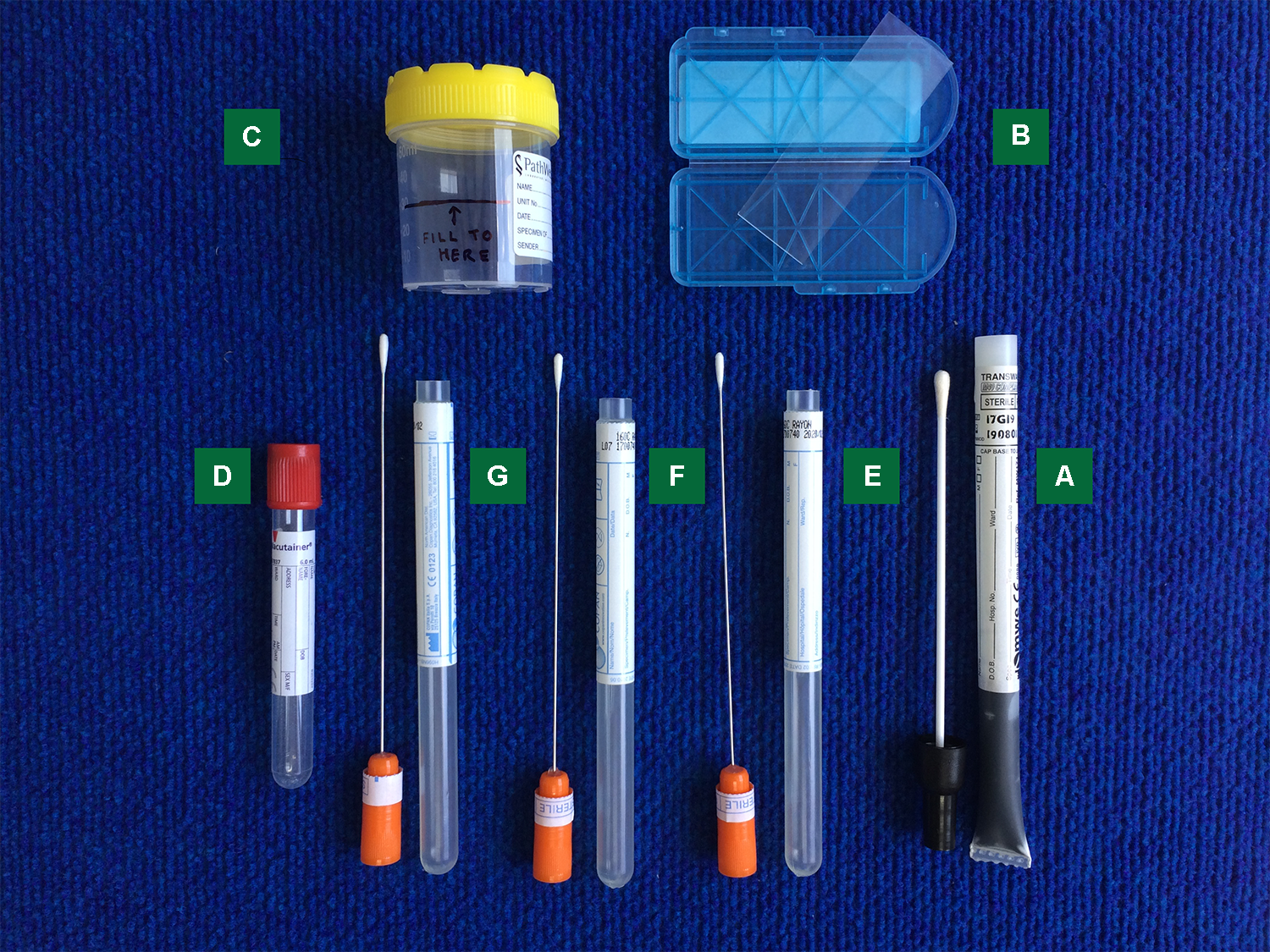
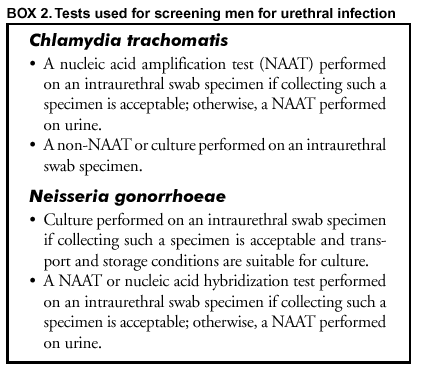
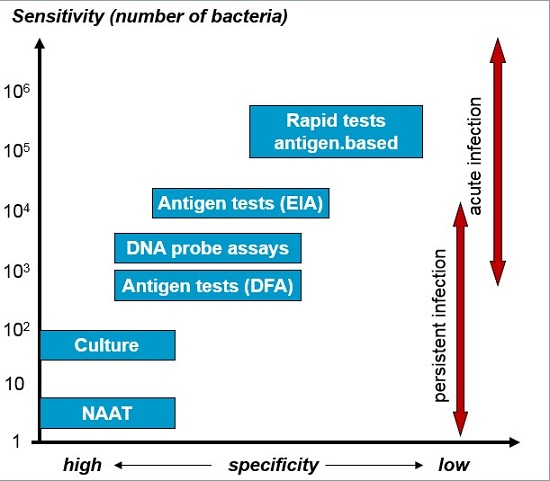
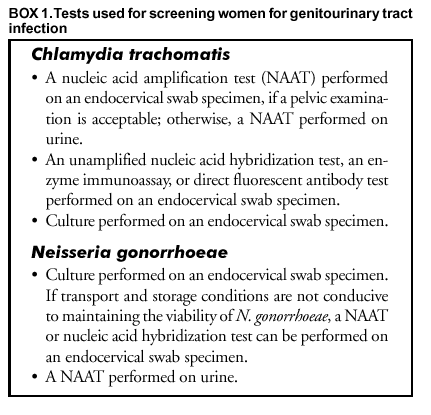
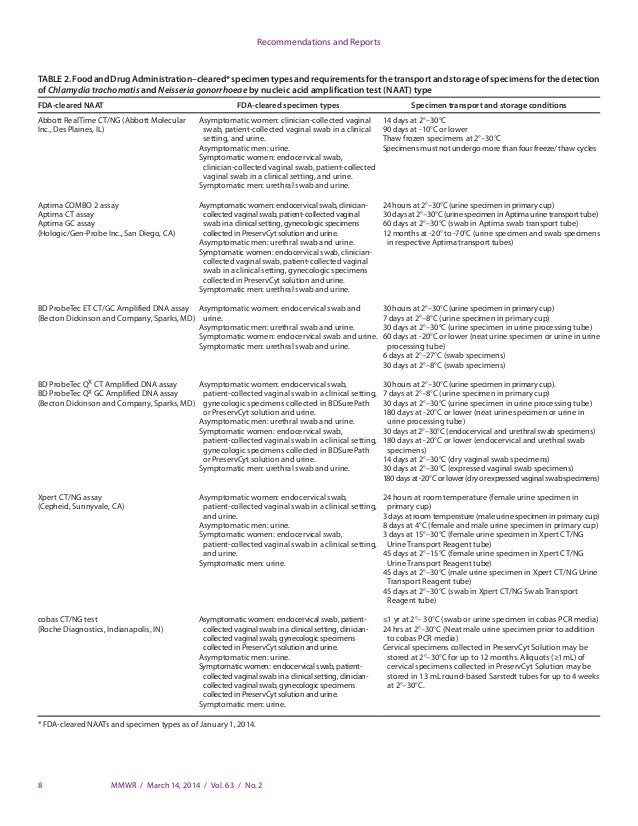
Endocervical Nucleic Acid Amplification Test Naat Swab
Any swab with a positive result of the first test was confirmed with a second naat specific for chlamydia.
Endocervical nucleic acid amplification test naat swab. Endocervical nucleic acid amplification test naat the endocervical naat swab should be performed first. An endocervical naat swab a high vaginal charcoal media swab and an endocervical charcoal media swab. Conventional agar based culture method and nucleic acid amplification test naat of the cppb gene for detection of neisseria gonorrhea in pregnant women endocervical swab specimens. This test is intended to be performed on respiratory specimens collected from individuals who meet the centers for diseases control and prevention cdc clinical and or.
Nasal or throat swab most tests saliva a few tests. The endocervical naat kit usually. Both swabs were tested using a nucleic acid amplification test naat designed to detect both chlamydia and gonorrhea. Hassanzadeh p 1 mardaneh j motamedifar m.
During examination the clinician obtained an endocervical swab. Chlamydia was diagnosed if either swab resulted in 2. The laboratory issues specific collection kits for chlamydia gc testing using the aptima hologic panther. Nucleic acid amplification test naat patient self take instructions.
Insert the specimen collection swab blue shaft into the endocervical canal. Rapid diagnostic test. The swabs are listed below in the order which you should take them. 1 department of biology school of sciences shiraz university shiraz ir iran.
Gently rotate the swab clockwise for 10 to 30 seconds in the endocervical canal to ensure adequate sampling. Sars cov 2 rna covid 19 qualitative naat the sars cov 2 rna covid 19 nucleic acid amplification test naat is a qualitative multi target molecular diagnostics test that aids in the detection of covid 19.